
It makes the metal soft and low-melting materials.
All the alkali metals like lithium, sodium, potassium, and rubidium are soft, low melting, silvery-white, metals but cesium is a golden yellow colour.Chlorine liberated by this production process at the central graphite electrode (anode) is collected through the nickel dome. It is preferentially deposited with 1 to 2 percent of calcium metal in a cylindrical steel cathode. Under such conditions, the discharge potential of the Na + ion is lower than that of the Ca +2 ion. Therefore, the difficulties from the volatility of Na (boiling point 883 ☌) are largely eliminated. The temperature for electrolysis is considerably lower than the melting point of pure sodium chloride (803 ☌). Sodium is made by the electrolysis of a fused mixture of NaCl and CaCl 2. Na + ion constitutes about 0.3 percent of human blood, 0.6 percent of bone, and 0.6 to 1.5 percent of body muscles. Plants and animal organism contains sodium compounds mainly NaCl. Besides the rock salts, natural brines and oceanic waters contain large amounts of sodium chloride. Similar deposits are found in Saskatchewan, Canada, and Carlsbad, New Mexico. The Cheshire salt field in the United Kingdom occupies areas of (60 km × 24 km) and a nearly 400-meter thick layer. The vast deposit of rock salt may be obtained by the evaporation of ancient seas.
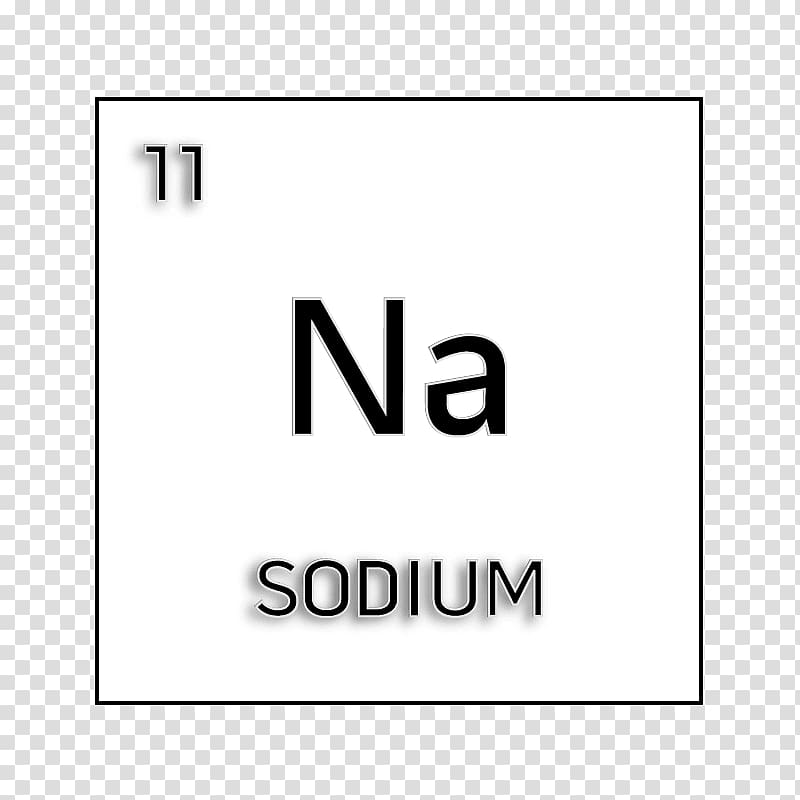
It has occurred in many minerals like rock salt (NaCl), carbonate (trona), nitrate (saltpeter), borate ( borax), etc. It has found 22,700 ppm in the earth’s crust. Sodium is the seventh most abundant periodic table element and the fifth most abundant metal after aluminum, iron, calcium, and magnesium. Some physical and chemical properties like melting point, boiling point, common oxidation number or state, and density are given below the table. The physical and chemical properties of the element are readily understood in terms of its simple outer electronic configuration. Due to the presence of 3s 1 outer electronic configuration, the alkali metal sodium occupies Group-1 or 1A on the periodic table. The electronic configuration of the alkali metal sodium is 1s 2 2s 2 2p 6 3s 1.


 0 kommentar(er)
0 kommentar(er)
